A new cellular type in invertebrates: first evidence of telocytes in leech Hirudo medicinalis
| Journal/Publisher | Scientific Reports |
|---|---|
| Year | 2017 |
| Authors | Laura Pulze, Nicolò Baranzini, Rossana Girardello, Annalisa Grimaldi, Lidia Ibba-Manneschi, Enzo Ottaviani, Marcella Reguzzoni, Gianluca Tettamanti & Magda de Eguileor |
| Link | https://www.nature.com/articles/s41598-017-13202-9#Sec8 |
| Dataset | [email protected] |
| Log Date | |
| Type | ArticlePeer-Review Record |
| Technique | |
| Dataset Raw |
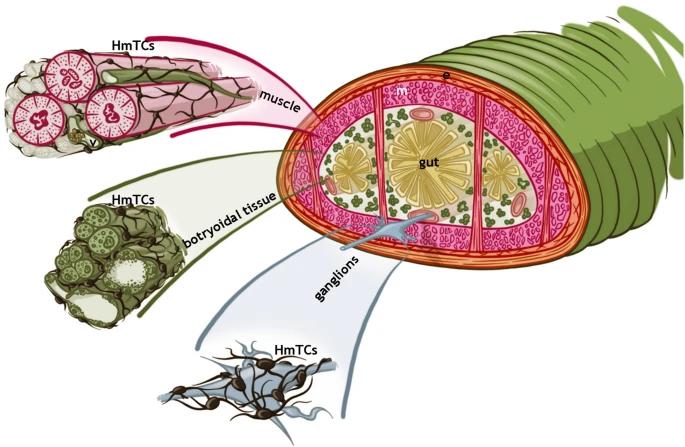
Figure 1
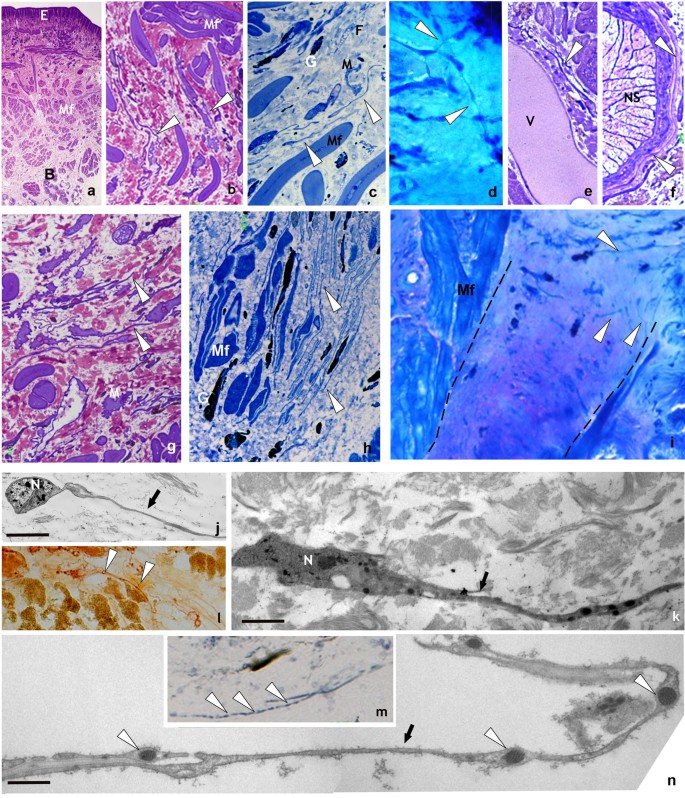
Unstimulated and stimulated H. medicinalis: optical and TEM microscopy. (a–f) Light microscopy, semi-thin sections of healthy (unstimulated) leech. (a) General view of cross sectioned body of leech (Crystal violet staining). Under the epithelium (E), the body wall is mainly composed of tightly packed helical muscle fibers (Mf) embedded in a scarce and loose connective tissue. Under the longitudinal layer of muscle fibers the botryoidal tissue is visible (B). (b) Detail of muscular body wall. Among muscle fibers (Mf), embedded in the loose connective tissue, interstitial cells showing telocyte phenotype are visible (white arrowheads). (c) Specimen stained with methylene blue, which is considered the best dye in identifying telocyte feature. In the connective tissue HmTCs, showing small cell bodies and thin long cellular processes (white arrowheads) are easily recognizable in respect to granulocytes (G), fibroblasts (F) and macrophages (M). (d) Specimen May-Grunwald Giemsa. HmTCs (arrowheads) in close contact by their tubular long cytoplasmic processes are embedded in the loose connective tissue. (e,f) Specimens Toluidine blue stained. HmTCs’ telopodes (arrowhead) surround a small vessel (V) and ganglia (nervous system-NS). (g–i) Semi-thin sections of leeches stimulated by LPS injection (g,h) or subjected to surgical lesion (i). In the specimens stained with crystal violet (g) and with methylene blue (h) telocytes (white arrowheads), wide distributed in the connective tissue, among muscle fibers (Mf) and granulocytes (G), are more numerous than controls. In leech surgically lesioned (specimen stained with May-Grunwald Giemsa) (i), the outlined wedge-shaped regenerating tissue is characterized by a large amount of newly synthesized connective tissue infiltrated with proliferating (expressing stemness factors) telocytes (arrowheads). (Mf: muscle fibers). (l,m) Cryosections of leech body wall. HmTCs (arrowheads) are Nonspecific esterase+ (NSE+) (l) and Succinic dehydrogenase+ (SDH+) (m). Nonspecific esterase reaction is linked to microsomal and lysosomal activity while SDH reaction is attributable to mitochondrial activity. Intense spotted SDH staining evidences mitochondria localized in podoms (arrowheads). (j,k,n) Ultrastructural analysis (TEM) confirm the telocyte phenotype characterized by spindle-shape cell body and long and thin telopods (arrows). Note the localization of mitochondria within podoms (n) (arrowheads) that validates the SDH moniliform positivity (m). Scale bars. j: 2 μm; k: 0.9 μm; n: 2.2 μm.
Figure 2
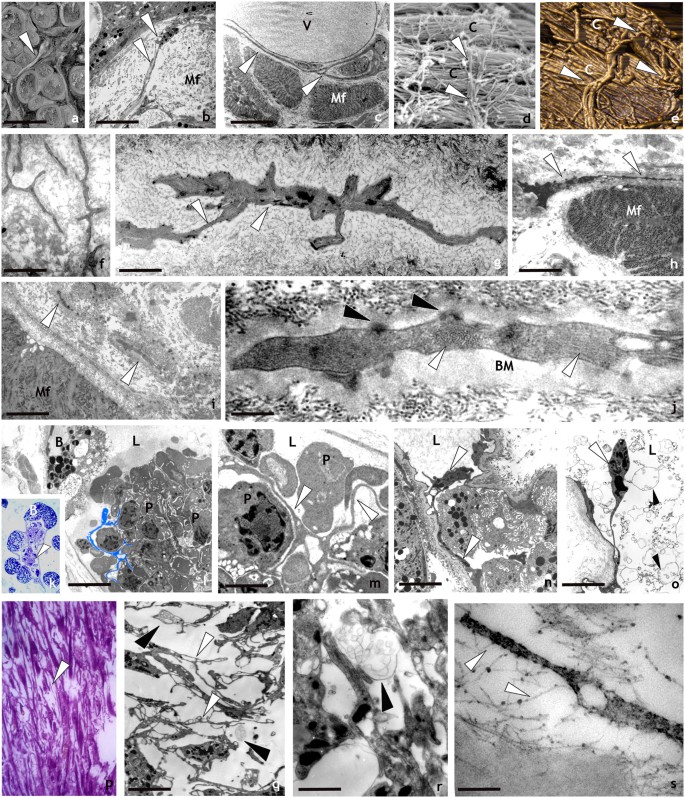
Spatial organization and behaviour of HmTCs after activation by LPS injection or surgical lesion revealed by optical, SEM, AFM and TEM analysis. (a–j) Ultrastructure: -SEM (a,d), TEM (b,c) and AFM (e) images of leech body wall: HmTCs (arrowheads) can be in close contact with muscle fibres (Mf), embrace vessels (V) with their telopodes or are orthogonally placed in respect to collagen bundles (c) as visible both in SEM and AFM photographs (d,e). Scale bars. b: 4 μm; c: 3 μm; f: 3 μm; g: 1.4 μm; h: 2 μm; i: 1.7 μm; j: 0.2 μm; l: 4 μm; m: 1 μm; n: 3 μm; o- 3 μm; q: 3 μm;r: 1.4 μm; s: 0.25 μm. (f–j) Thin sections: HmTCs embedded in the connective tissue, acquire the phenotype typical of migrating cells showing the cytoplasm filled with filament bundles perpendicularly oriented (j) (white arrowheads). Hemidesmosomes (j) (black arrowheads) are visible in connection with a thick basal membrane (BM). (k–o) HmTCs in angiogenic process: (k) semithin section of botryoidal (B) and endothelial cells shaping a new lumen (L) in which clusters of circulating precursor cells are visible (arrowhead). At ultrastructural level (l–o), HmTCs (some of them false-coloured in light blue) (l) are visible among circulating precursor cells (P) inside the neo-vessel (L: lumen). (m) Detail of relationship among branched HmTCs and circulating precursor cells (P). HmTC (n) with long telopode (arrowheads) in the capillary lumen (L). (o) HmTC “floating” (white arrowhead) in the lumen (L), is surrounded by numerous multivesicular bodies (black arrowheads). (p–s) Semithin and thin sections of leech wound healing. The regenerating tissue is characterised by a large amount of migrated cells. Among them, HmTCs (arrowheads) (p,q,r) engage numerous direct interaction with adjacent cells (q) and/or with collagen (s) via proteoglycans (white arrowheads). Close to HmTCs multivesicular bodies are visible (q,r) (black arrowheads).
Figure 3
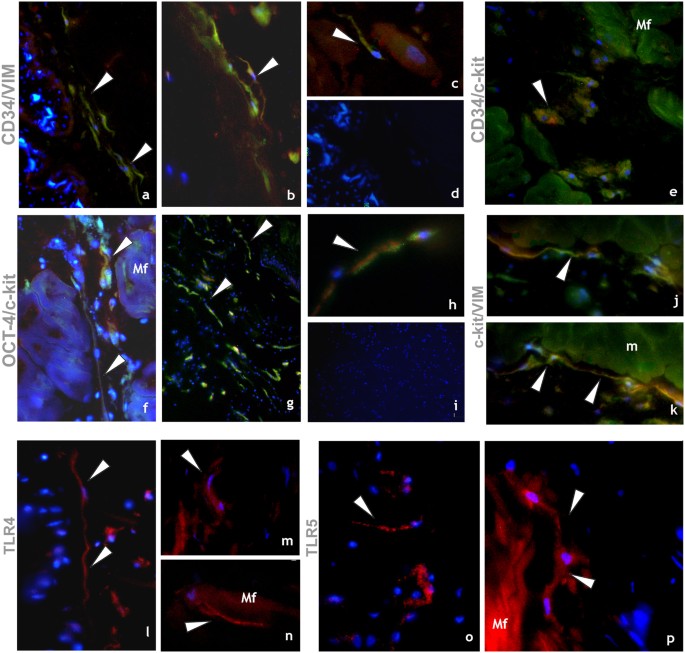
Immunocytochemical characterization of HmTCs. (a–p) Cryosections of leech activated body wall: (a–k) Double-immunostainings proposed as appropriate to identify telocytes. Samples stained by using antibodies against CD34 (green)/vimentin (red) (a,d); CD34 (green)/c-kit (red) (e); Oct-4 (green)/c-kit (red) (f–h); c-kit (green)/vimentin (red) (j,k) reveal cells with typical phenotype of telocytes (arrowheads). The two signals co-localize (merge in yellow) (arrowheads). (d,i) Negative controls. Nuclei are counterstained with DAPI and marked in brilliant blue. (l–p) HmTCs, wide spread in the body muscular sac (Mf), precisely identified for their morphology, are characterized by expression of TLRs (arrowheads). TLR4 (l–n) and TLR5 (o,p) that are evolutionarily conserved receptors playing a critical role in the early innate immune responses.
Figure 4
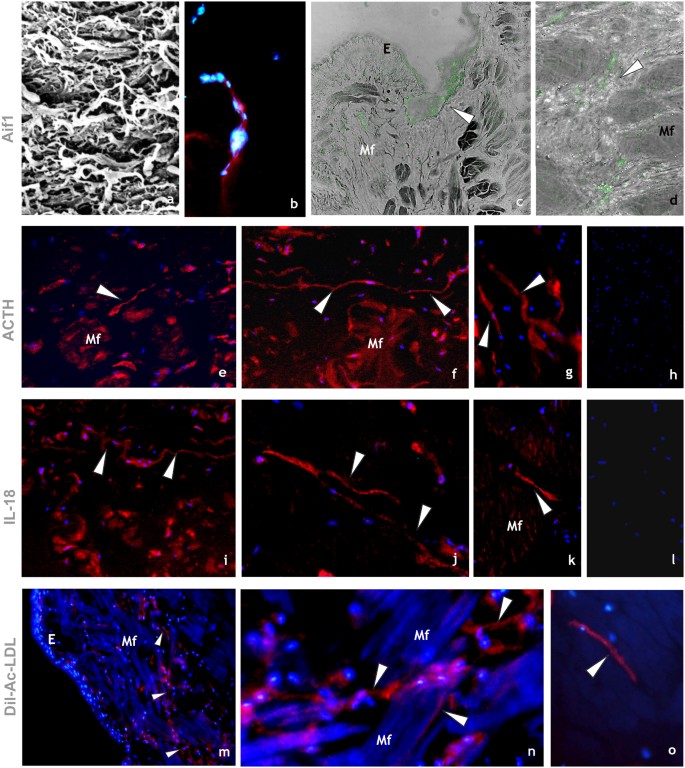
Immunocytochemical characterization of HmTCs. (a–d) HmTCs, recruited into lesioned area together with various cell types involved in migration and wound restoring, show own ultrastructural (SEM) profile (a). Cryosections of leech activated body wall: HmTCs are Aif-1 positive (red) (b). Nuclei are counterstained with DAPI (blue). (c,d) Immune-responsive cells (not exclusively belonging to telocytes) located under the epithelium (E) and among muscle fibres (Mf) are Aif-1 positive (green). Aif-1, also known as IBA1, is identified as a modulator in immune defence reaction and inflammatory response. (e–g) HmTCs (arrowheads) localized among muscle fibres (Mf), recognizable for their spindle-shaped body and long cellular prolongations, are ACTH positive. ACTH expression is signal of activation of stress-sensoring circuits. Not only telocytes positively bound antibody anti ACTH but these stromal cells are always identifiable for their morphology. (h) Negative control. Nuclei are stained with DAPI and marked in brilliant blue. (i–k) Cryosections of leech activated. HmTCs recognizable among various cell types (arrowheads) are IL-18 positive. The production of this representative pro-inflammatory cytokine is generally linked with activating innate immunity. (l) Negative control. Nuclei are stained with DAPI. (m–o) Cryosections of leech activated. DIL-ac-LDL uptake is considered a good tool to define the origin and the fate of hematopoietic progenitor cells. HmTCs, DIL-ac-LDL positive (arrowheads), can migrate from the lumen of neovessels (see Fig. 3 panels k–o), travelling in the connective tissue, among muscle fibers (Mf).
The present paper provides evidence for the presence of TCs in the leech H. medicinalis which in this context is considered a good invertebrate animal model. This organism shows a “parenchimatous” body in which the loose connective tissue is colonized by several and different types of stromal cells such as macrophages, fibroblasts, myo-fibroblasts, NK-like cells and granulocytes, that share the same phenotype and function closely to the vertebrate counterpart. In addition, the basic steps of cellular responses, evoked by different stimuli, are strikingly similar to those observed in higher forms of life.
Among the leech stromal cells, the HmTCs can be detected due to their affinity for specific vital dyes. In non-stimulated animals, HmTCs in resting condition show the typical morphology described for vertebrate TCs: a pyriform or small ovoidal cell body with very long and thin cytoplasmic processes elongated up to 50 μm that are occupied by mitochondria, bunches of filaments perpendicularly oriented and few granules. The HmTC branches extend them in different directions and are engaged in cell-cell and cell-matrix contacts. The HmTCs form a network interconnecting cells from muscle layers, vessels, nervous system as well as collagenous bundles of connective tissue filling the leech body (Fig. 5, explanatory drawing). Once activated (after injection of LPS or following surgical lesion), these cells, organized in a plastic and dynamic system, can change their phenotype, acquiring an elongated shape suitable for migration with shorter telopods characterized by the involvement of the cytoskeletal elements.
Figure 5

Schematic overview explaining the spatial organization of HmTCs in Hirudo medicinalis. Telocytes (HmTCs) forming a 3D network connect cells from muscular, botryoidal, nervous and connective tissues.
HmTCs in healthy and in activated leeches are identified, as in vertebrate (i.e. in human) by double-positive immunostaining with CD34/vimentin, c-kit/vimentin, c-kit/CD34 and c-kit/Oct-4 which are stem cell markers expressed simultaneously with the hallmarks of TCs.
It is important to underline that another important characteristic of HmTCs is that they can recognize microbial components through TLRs that mediate activation of innate immunity. The detection of the non-self results in the inflammation phase with cell surface activation molecules followed by secretion of pro-inflammatory cytokines such as IL-18. These multifunctional cells, as several innate immune cells, express also HmAif-1, other factor typically involved in inflammatory responses52,53.
HmTCs, similarly to the major inflammatory cell types (i.e. immunocytes), are equipped to produce hormones such as ACTH and are able to respond to them. In our previous studies we have demonstrated that ACTH loop (controlling locally immune reactivity and regulating the cellular functions of the immune system in an autocrine and paracrine manner) has a key role in mutually influencing immune and neuroendocrine functions44,45,56,57,58,59,60.
The HmTC complex, that we defined as a “system in the system”, represents the most primitive immune organization, participating and conducting the first line of tissue/organism defences. The powerful ability of HmTC stromal network is due to the 3D organization that guarantees cross-talk among various cell types via intercellular contacts, secretion of cytokines and release of extracellular vesicles and exosomes.
These cells share with the various types of immunocytes the same mesodermal lineage as evidenced by their presence, after stimulation, inside the lumen of neo-vessels where are strictly connected with leukocyte-like cells. Experiments with the vital marker Dil-Ac-LDL, which is taken up preferentially by endothelial cells and precursor cells, are useful for staining and following the fate of these cellular types and to confirm the presence of HmTCs localized among precursors of circulating cells, within the lumen of newly formed vessels and dispersed among the groups of muscle cells or localized close to the injured region in wounded leeches. This finding reinforced the concept that hematopoietic precursor cells and telocytes, both of which develop in close association, have a mesenchymal common origin as in vertebrates.
Since HmTCs configure a 3D network of resident sentinels embedded in the connective tissue matrix, they can detect the first sign of non-self presence, and are required for proper communication among different cell types in all regions of the body. The HmTC immuno-surveillance system, obtained by direct cell-cell contacts and by soluble mediators released in loco, controls and directs the activity of neighbouring body cells. Once activated, the first response of HmTCs results in an alert centripetally and centrifugally directed. These communication precede the responses of integrated systems (i.e. cellular and soluble molecules) that the organism utilizes against any type of non-self. Moreover HmTC cross-talk by-passes both the slowness of the other types of innate immune cells that have to migrate and increase numerically before becoming operative and the slower spread, via matrix, of soluble molecules (growth factors and cytokines) that are produced by those cells directly involved in lesion or injected sites.
Thanks to the characteristics of stem cell, HmTCs are also engaged in regenerative processes at the lesion area.
Overall detailed analysis demonstrates that HmTCs are organized in a primitive ancient 3D system, part of innate immunity both in invertebrates and vertebrates. The fact that invertebrates and vertebrates could share cells, molecules and related functions inserted on a basic frame is not surprising according to an economic, conservative strategy in which a basic cellular network can provide the first line of body/tissue defence followed by more specific responses.
Moreover, due to the features of HmTC network we extend the list of leech defense mechanisms by addressing the problem of immunological memory in invertebrates. We have already suggested the existence of a kind of positive immune memory in allo- and xeno-transplantation in leeches40 and demonstrated that in second-set grafting experiments, responses to the second transplant were always faster and stronger than those occurring in first-set grafting experiments. Now we hypothesize that this form of innate immune memory (trained immunity) in invertebrate, unrelated to antibodies that mediate adaptive immunity, could be explained by how with the HmTCs’ function.
Indeed, a provocative thought arises from the intriguing comparison of HmTC network with microglia cells, not only in leeches but also in vertebrates. Interestingly microglia and TCs can be considered as the ancient conserved interface-system between immune and neuroendocrine function because share similar mediators and receptors from lower invertebrate up to human61,62,63,64,65,66,67. Support for this idea comes from comparative studies showing that TCs and microglia from H. medicinalis and vertebrates present a number of similarities in morphology, cellular functions, cell surface antigens, histochemical properties, mesodermal origin, capability to migrate, proliferate and differentiate in response to injury. It could be of interest to evaluate the possibility that TCs and microglial cells could represent the same type of cell resident in different districts able to detect the first signs of non-self invasion or tissue damage.